All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Impact of lysosomal acid lipase inhibition in controlling GvHD
Fatty acid metabolism has been implicated in graft-versus-host disease (GvHD) development after allogeneic hematopoietic cell transplantation. Oxidation of fatty acids in mitochondria is responsible for the generation of alloreactive T cells and blocking fatty acid oxidation leads to apoptosis of alloreactive T cells. Supply of fatty acids for oxidation is regulated by a number of enzymes. One of them is the lysosomal acid lipase (LAL) that induces hydrolysis of triglycerides in lysosomes. LAL has shown to be required for the function of a variety of cells, such as T cells, where its absence impairs T-cell receptor activation, T-cell proliferation, and cytokine secretion. Furthermore, studies have shown that LAL deficiency reduces T helper 1 and 2 cell differentiation while increasing the generation of regulatory T cells (Treg), supporting the hypothesis that LAL targeting may be beneficial for controlling GvHD.
Hung D. Nguyen and colleagues investigated the effects of LAL inhibition and deficiency on T-cell responses in mice models of leukemia. The results of the study were recently published in Cell Reports.1
Results1
LAL deficiency effects on T cells:
- Genetic LAL deficiency in CD4+ and CD8+ T cells negatively affected cell survival, proliferation, and activation, compared with wild-type counterparts in an in vitro mixed lymphocyte reaction using LAL+/+ and LAL−/− T cells from FVB mice, and T cell-depleted (TCD) splenocytes from BALB/c mice
- LAL-deficient T cells showed
- increased necrosis
- decreased proliferation
- lower levels of proinflammatory cytokine production, such as interferon-gamma and tumor necrosis factor-alpha
- decreased fatty acid oxidation
- The impact of LAL deficiency was strongest in LAL-deficient CD4+ T cells, and less profound in CD8+ T cells
- Using fat-free medium led to T-cell dysfunction in wild-type T cells, shown by reduced proliferation and activation, while improving T-cell function in LAL-deficient T cells. This indicates that T-cell activation is facilitated by fatty acids, but the relative contribution is mediated through LAL
LAL deficiency reduces GvHD severity while maintaining graft-versus-tumor (GvT) activity
Transplanted LAL-deficient donor T cells induced lower GvHD scores and improved survival in two bone marrow transplant models (major histocompatibility complex-mismatched B6 [H2b] → BALB/c [H2d], and FVB [H2q] → B6 [H2b]).
Additionally, LAL-deficient T cells retained sufficient activity to mediate GvT response as evidenced using a haploidentical B6 (H2b)/BDF1 (H2b/d) bone marrow transplantation model transplanted with luciferase-transduced P815 mastocytoma cells:
- The recipients transplanted with TCD bone marrow cells and LAL-wild-type T cells died without signs of tumor relapse within 20 days from the transplant, but displayed significant GvHD severity
- Half of recipients transplanted with TCD bone marrow cells and LAL-deficient T cells were still alive 60 days after transplant, most of them tumor-free, and without clinical signs of GvHD
Pharmacological inhibition of LAL prevents GvHD
Similar to LAL deficiency, pharmacological inhibition of LAL using orlistat led to significant effects on T cells, such as
- reduced proliferation (decreased Ki67 expression)
- decreased activation (reduced production of interferon-gamma)
- reduced fatty acid metabolism (low Cpt1a expression)
- increased Foxp3 expression
LAL inhibition with orlistat also prevented GvHD in bone marrow transplant recipients, indicated by improved survival and lower clinical scores. Doses between 8 and 20 mg/kg/day for 14 days were found to be nontoxic.
Additionally, orlistat treatment maintained GvT effects in two mice models. In one model (B6 → BDF1 model transplanted with P815 mastocytoma cells), LAL inhibition with orlistat rescued 40% of bone marrow transplant recipients from GvHD mortality and tumor relapse, which was confirmed with similar results in a second model.
LAL deficiency has different effects on spleen and gut T cells
LAL deficiency affects T cells differentially in the spleen and gut in the development of GvHD (see Figure 1):
- Spleen — 7 days after bone marrow transplant, LAL-deficient donor CD4+ T cells, but not CD8+, showed significantly reduced proliferation, migration potential, and intercellular lipid content, but increased Treg generation in recipient spleens. The effects of orlistat treatment were similar to LAL deficiency, except for the lack of Treg generation
- Gut — In recipient guts, LAL-deficient donor T cells were significantly decreased, likely due to impaired survival and/or migration. This effect was particularly pronounced in CD4+ T cells:
- consistent with results observed in the spleen, Treg generation was also increased in LAL−/− CD4 T cells in recipient guts
- however, both donor CD4+ and CD8+ T cells had significantly increased intracellular lipid content, reactive oxygen species generation, and levels of T-cell exhaustion
Overall, the treatment with orlistat simulated LAL deficiency. However, orlistat treatment failed to increase Treg generation from donor T cells.
Figure 1. LAL deficiency and orlistat effects on donor T cells in recipient spleens and guts1
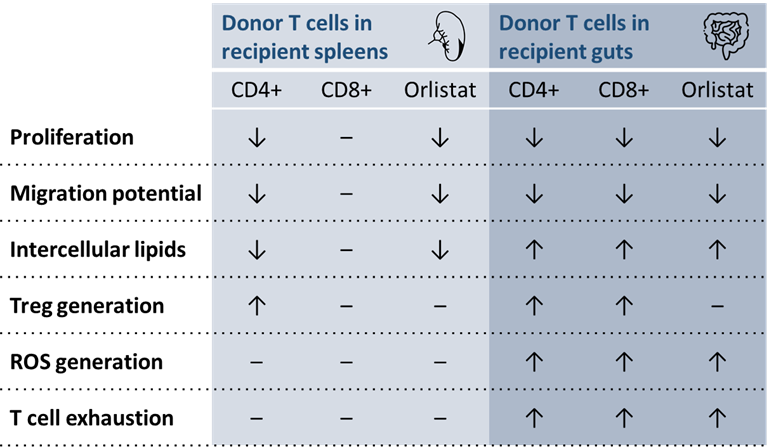
LAL, lysosomal acid lipase; ROS, reactive oxygen species.
↑ increase; ↓ decrease; – no change. All increases and decreases were significant.
Conclusion
- LAL activity is important for donor T-cell survival, metabolism, and function
- In recipient spleens, LAL deficiency seems to reduce T-cell migration while maintaining T-cell activation and metabolism
- In recipient guts, however, LAL deficiency increased Treg generation and decreased donor T-cell activation, differentiation, and migration, thereby reducing GvHD pathology
- LAL deficient T cells maintain GvT activity in transplant models
- Similarly, pharmacological inhibition of LAL using orlistat can effectively prevent GvHD, while still largely preserving the GvT activity of donor T cells. However, orlistat does not induce Treg generation
- LAL may be a promising target for the control of GvHD and tumor relapse after allogeneic hematopoietic cell transplantation
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

